Chemistry Is an Imaginative World To a highly enthusiastic person with courage of experiment and exploration.
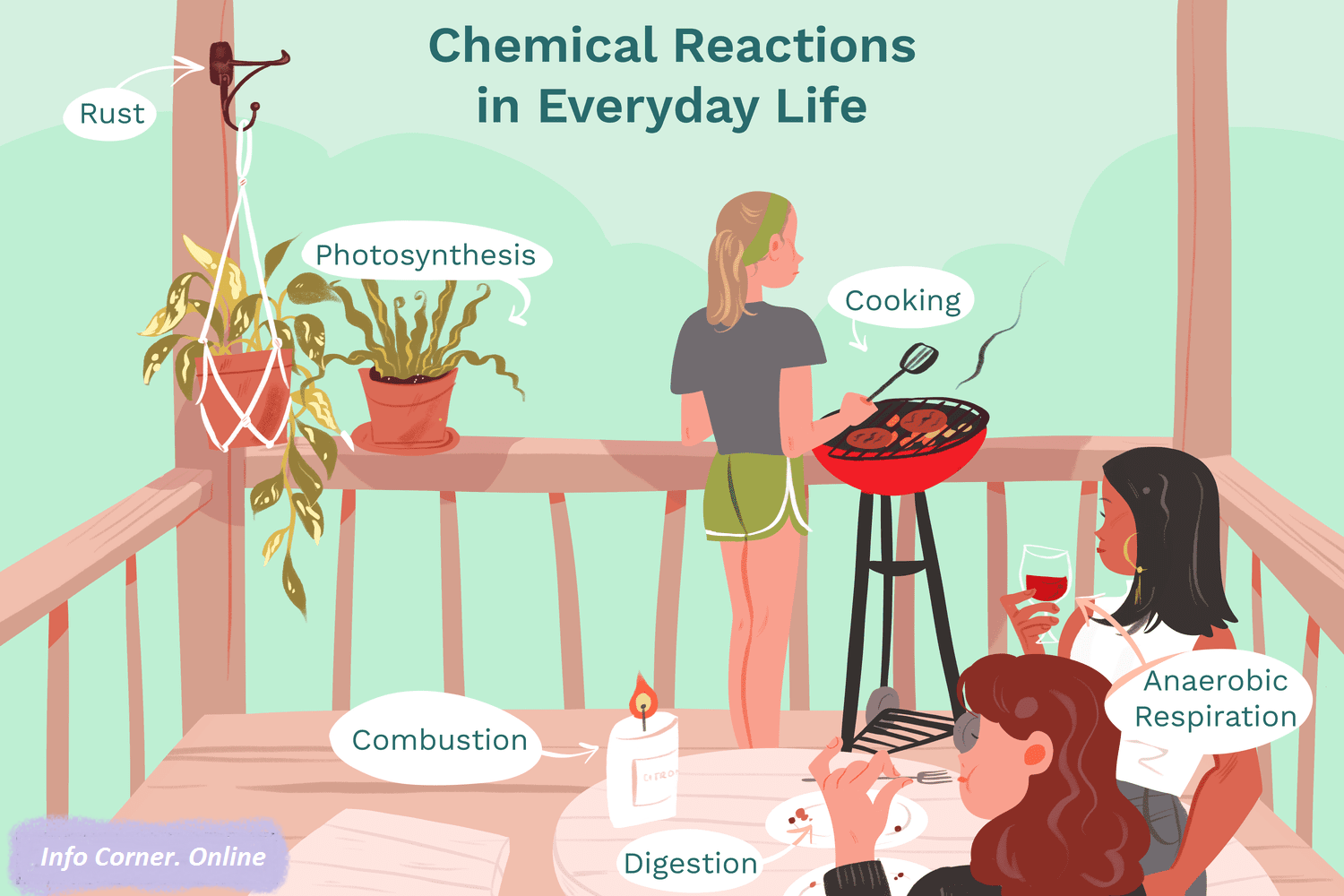
Rusting/Corrosion of Iron

Introduction to the Rusting of Iron
The rusting of iron is a chemical process that leads to the deterioration of iron and its alloys. This phenomenon, commonly known as corrosion, has significant implications in various industries, including construction, automotive, and manufacturing. Understanding the rusting process is crucial for developing effective strategies to prevent and control it.
Causes of Iron Rusting
Rusting is primarily caused by the reaction of iron with oxygen and moisture in the environment. When iron is exposed to water and oxygen, it forms iron oxide, commonly known as rust. Factors such as humidity, saltwater, and pollutants can accelerate this process. The presence of electrolytes, such as salt, enhances the conductivity of water, facilitating the electrochemical reactions that lead to rust formation.
The Rusting Process
The rusting of iron involves a series of electrochemical reactions. Initially, iron reacts with water to form iron hydroxide. This compound subsequently reacts with oxygen to produce iron oxide. The formation of rust weakens the metal, causing it to flake away over time. The overall process can be described by the following reactions:
1. Fe + H2O → Fe(OH)2
2. Fe(OH)2 + O2 → Fe2O3•nH2O (rust)
Preventing Rusting
Preventing the rusting of iron is essential for maintaining the integrity and longevity of metal structures. Several methods can be employed to protect iron from corrosion:
1. **Coating and Painting:** Applying protective coatings, such as paint, oil, or galvanizing, creates a barrier that prevents moisture and oxygen from reaching the metal surface.
2. **Cathodic Protection:** This technique involves using a sacrificial anode, typically made of a more reactive metal, to divert the corrosion process away from the iron.
3. **Environmental Control:** Reducing exposure to moisture, pollutants, and electrolytes can significantly slow down the rusting process.
Conclusion
Understanding the causes and mechanisms of iron rusting is vital for developing effective prevention strategies. By implementing protective measures, industries can mitigate the adverse effects of corrosion, ensuring the durability and safety of iron-based structures.
Benefits of Sunscreen

Introduction to Sunscreen
Sunscreen is a crucial element in our daily skincare regimen, often overlooked yet significantly beneficial. Its primary function is to protect our skin from the harmful effects of ultraviolet (UV) rays emitted by the sun. This protection is essential to maintain healthy skin and prevent various skin-related issues.
Protection from UV Radiation
Ultraviolet radiation is a key factor contributing to skin damage. Prolonged exposure can lead to sunburn, premature aging, and in severe cases, skin cancer. Sunscreen acts as a barrier, absorbing or reflecting these harmful rays, thereby minimizing their impact on the skin. By incorporating sunscreen into our daily routine, we can significantly reduce the risk of these adverse effects.
Preventing Premature Aging
One of the most noticeable benefits of using sunscreen is its ability to prevent premature aging. UV rays accelerate the aging process by breaking down collagen and elastin in the skin, leading to wrinkles, fine lines, and age spots. Regular application of sunscreen helps maintain the skin’s youthful appearance by protecting these vital components, ensuring that the skin remains firm and elastic.
Maintaining Even Skin Tone
Uneven skin tone and hyperpigmentation are common issues caused by sun exposure. Sunscreen helps in preventing these concerns by protecting the skin from UV-induced damage. Consistent use of sunscreen can lead to a more even and balanced complexion, free from dark spots and discoloration.
Conclusion
In conclusion, the benefits of sunscreen extend beyond mere protection from sunburn. It plays a vital role in safeguarding our skin from various forms of damage, including premature aging and uneven skin tone. Incorporating sunscreen into our daily skincare routine is a simple yet effective way to maintain healthy, youthful, and radiant skin.
Solar Cell Technology as Renewable Energy Source
Introduction to Solar Cells
Solar cells, also known as photovoltaic (PV) cells, are devices that convert light energy into electrical energy through the photovoltaic effect. This technology plays a crucial role in harnessing renewable energy, significantly contributing to sustainable development. Understanding the chemistry behind solar cells can provide insight into their functionality and efficiency.
The Photovoltaic Effect
The core principle behind solar cells is the photovoltaic effect, a process where light photons are absorbed by a material, causing the excitation of electrons. These excited electrons create an electric current when they move through the material. The primary material used in most solar cells is silicon, a semiconductor that efficiently facilitates this process.
Role of Semiconductors
Semiconductors like silicon are fundamental to the operation of solar cells. In their pure form, semiconductors have limited conductivity. However, through a process called doping, impurities are added to enhance their conductive properties. This doping process creates two types of semiconductors: n-type (negative) and p-type (positive). The junction between these two types, known as the p-n junction, is where the photovoltaic effect occurs.
Generation of Electric Current
When sunlight hits the solar cell, photons are absorbed by the semiconductor material, providing energy to electrons and allowing them to break free from their atomic bonds. These free electrons move towards the n-type layer, while the holes (the absence of electrons) move towards the p-type layer. This separation of charge carriers creates an electric field at the p-n junction, generating a voltage. When an external circuit is connected, this voltage drives an electric current, powering devices or storing energy.
Conclusion
The chemistry behind solar cells is a fascinating interplay of materials science and physics. By understanding the underlying chemical processes, we can appreciate the sophistication and potential of solar technology in providing a sustainable energy solution. Continued research and development in this field promise even more efficient and cost-effective solar cells in the future.
She is a Master of Philosopher in Chemistry from the University of Agriculture Faisalabad, Pakistan. year 2016. She is serving as a Teacher in Public Sector since 2015. She is also Masters in Education.
Proudly powered by WordPress

